siiky
2023/07/28
2023/12/05
2023/12/05
science,biology
Most of organic chemistry—the chemistry of carbon-containing compounds, which are central to biology—involves interactions between electrons in s and p subshells, so these are the most important subshell types to be familiar with.
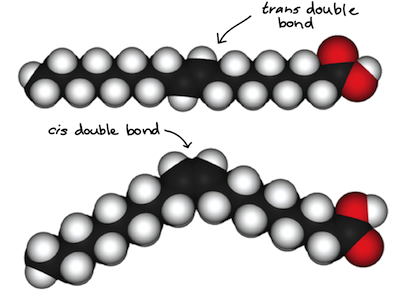
In fats and oils, long carbon chains called fatty acids often contain double bonds, which can be in either the cis or trans configuration (shown below). Fatty acids that contain cis double bonds are typically oils at room temperature. This is because the bends in the backbone caused by cis double bonds prevent the fatty acids from packing tightly together. In contrast, fatty acids with trans double bonds (popularly called trans fats), are relatively linear, so they can pack tightly together at room temperature and form solid fats.
Trans fats are linked to an increased risk of cardiovascular disease, so many food manufacturers have eliminated their use in recent years. Fats with trans double bonds are found in some types of shortening and margarine, while fats with cis double bonds may be found in oils, such as olive oil and canola oil. See the article on lipids to learn more about the different types of fats.
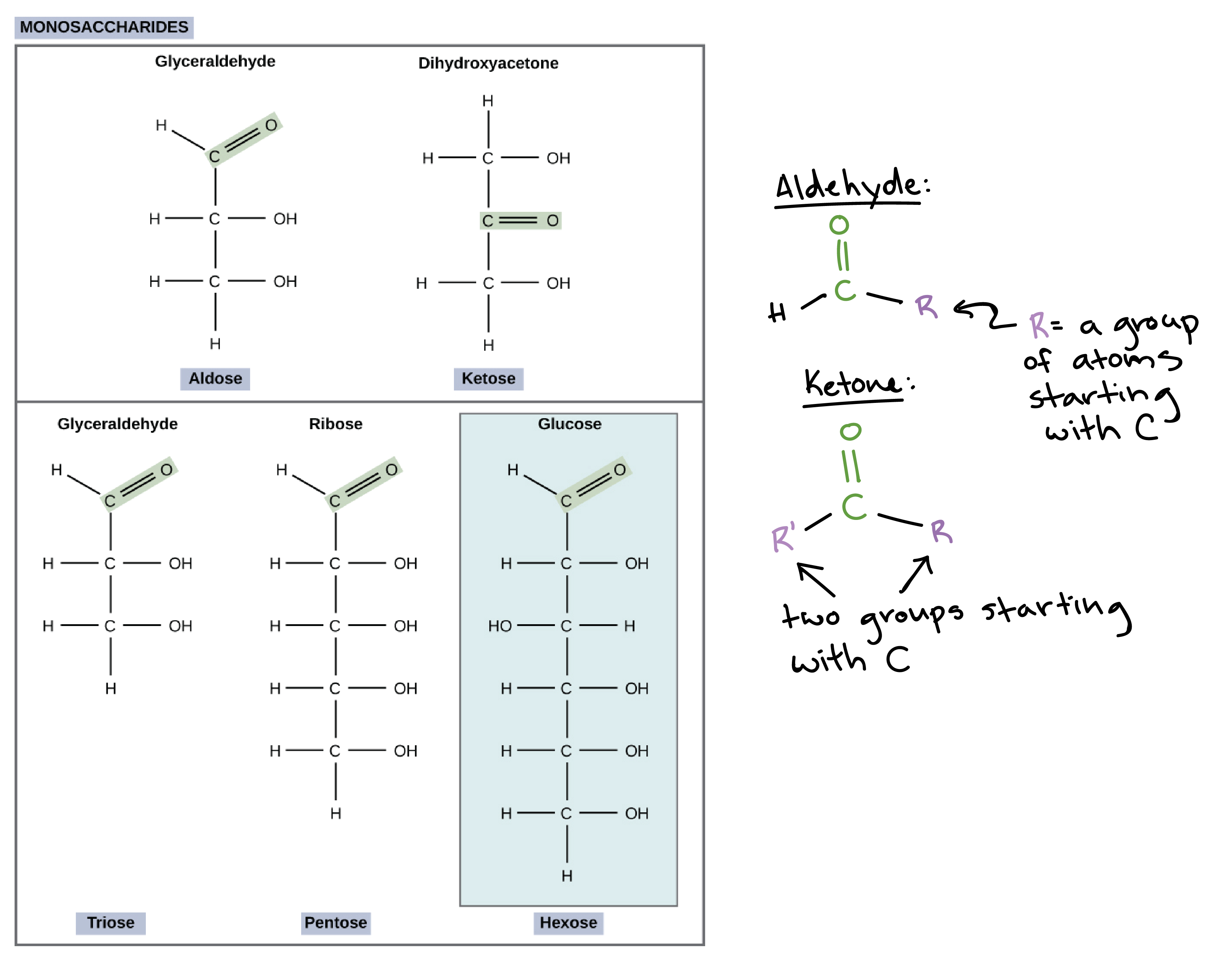
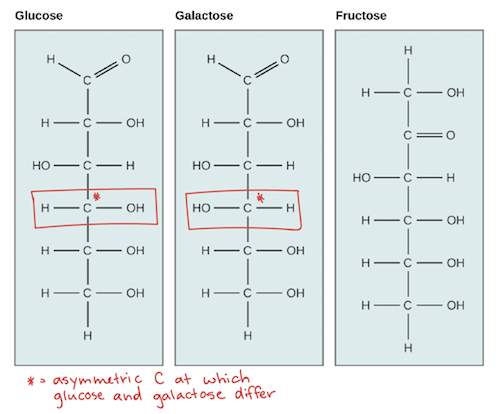
Glucose, Galactose, and Fructose are C6H12O6 isomers!
Sucrose ("table sugar") is a disaccharide made of one glucose and one fructose monomer. A disaccharide is a pair of monosaccharides joined together through dehydration synthesis (aka condensation reaction). The following image pictures the dehydration synthesis of a glucose and a fructose into a sucrose (the dehydration synthesis process releases an H2O molecule, though it isn't pictured).
Lactose is also a sugar (never noticed the similarity in name), a disaccharide of glucose and galactose. And Maltose is a disaccharide of two glucose molecules.
The inverse process of dehydration synthesis, which splits a di- or polysaccharide into a glucose and a smaller di- or polysaccharide (two glucoses in the case of a disaccharide, of course), is called hydrolisis. Because it's the inverse process, it uses up one H2O molecule.
A very interesting thing is that cellulose -- the composition of paper, wood, &c -- is also a polysaccharide! It's actually a long unbranched chain of glucose. These long chains can bundle up together, in a parallel fashion, through hydrogen bonding, and thereby resulting in the solidity of wood and other plant tissues. Even though cellulose is basically just glucose, humans can't digest it because they're of the β variety. This doesn't make cellulose bad for us, we just don't digest it.
Another important polysaccharide is chitin, the main constituent of the exoskeleton of arthropods (e.g. insects, arachnids, crustaceans).